Collaborations with the Army Research Lab (DEVCOM, CBC) seek to understand the photochemical mechanisms of MOF photocatalysis.
Chemical warfare agents (CWAs) can spread rapidly and will contaminate their
surroundings if left unchecked. To those who are exposed to CWAs, reusable protective
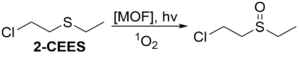
equipment would be critical for their safety. It is hypothesized that the use of polymer-bound metal-organic frameworks (MOFs) can catalyze the breakdown of sulfur mustard,
a deadly CWA. The MOF produces singlet oxygen which oxidizes sulfur mustard upon
exposure to UV light and repeatedly undergoes this reaction when embedded into a
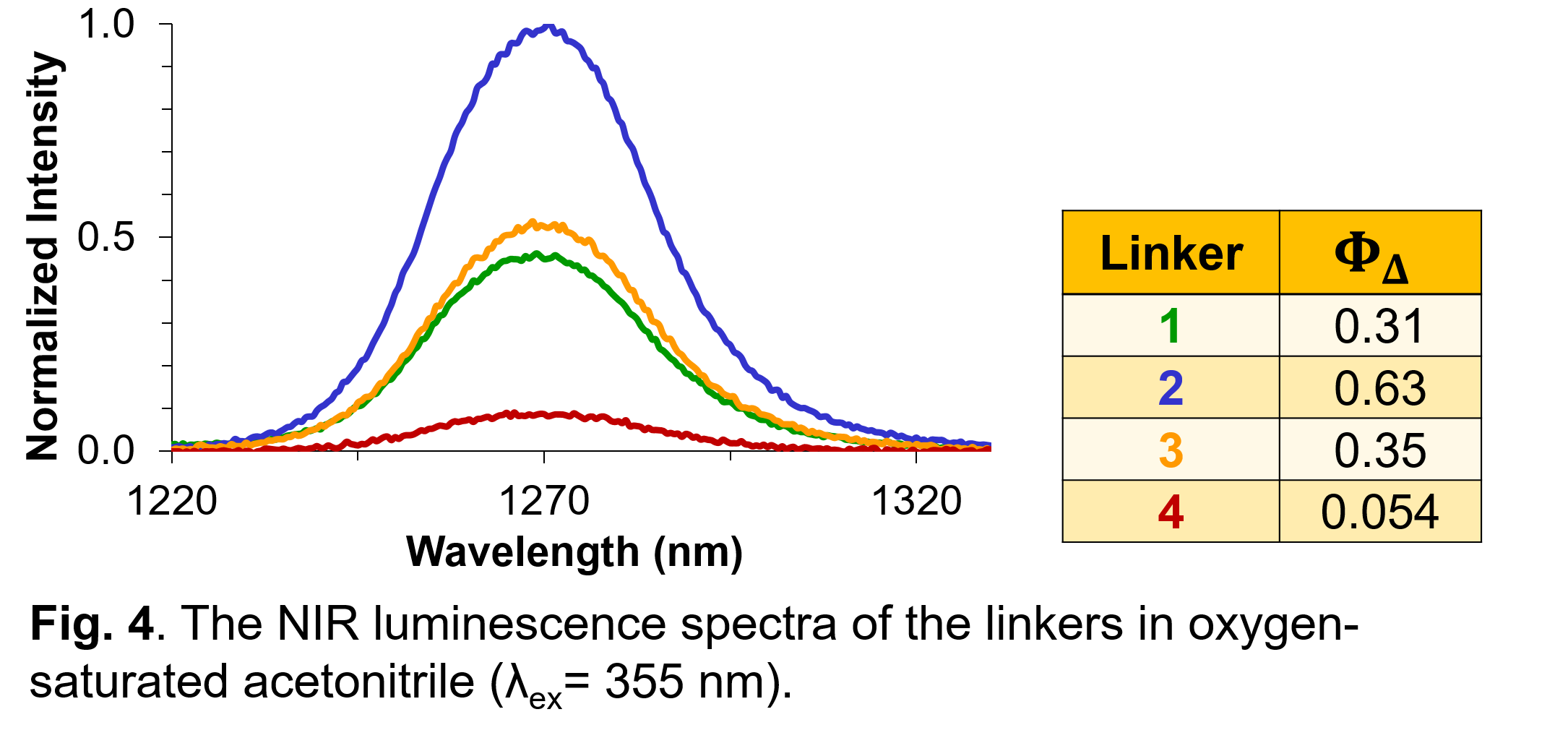
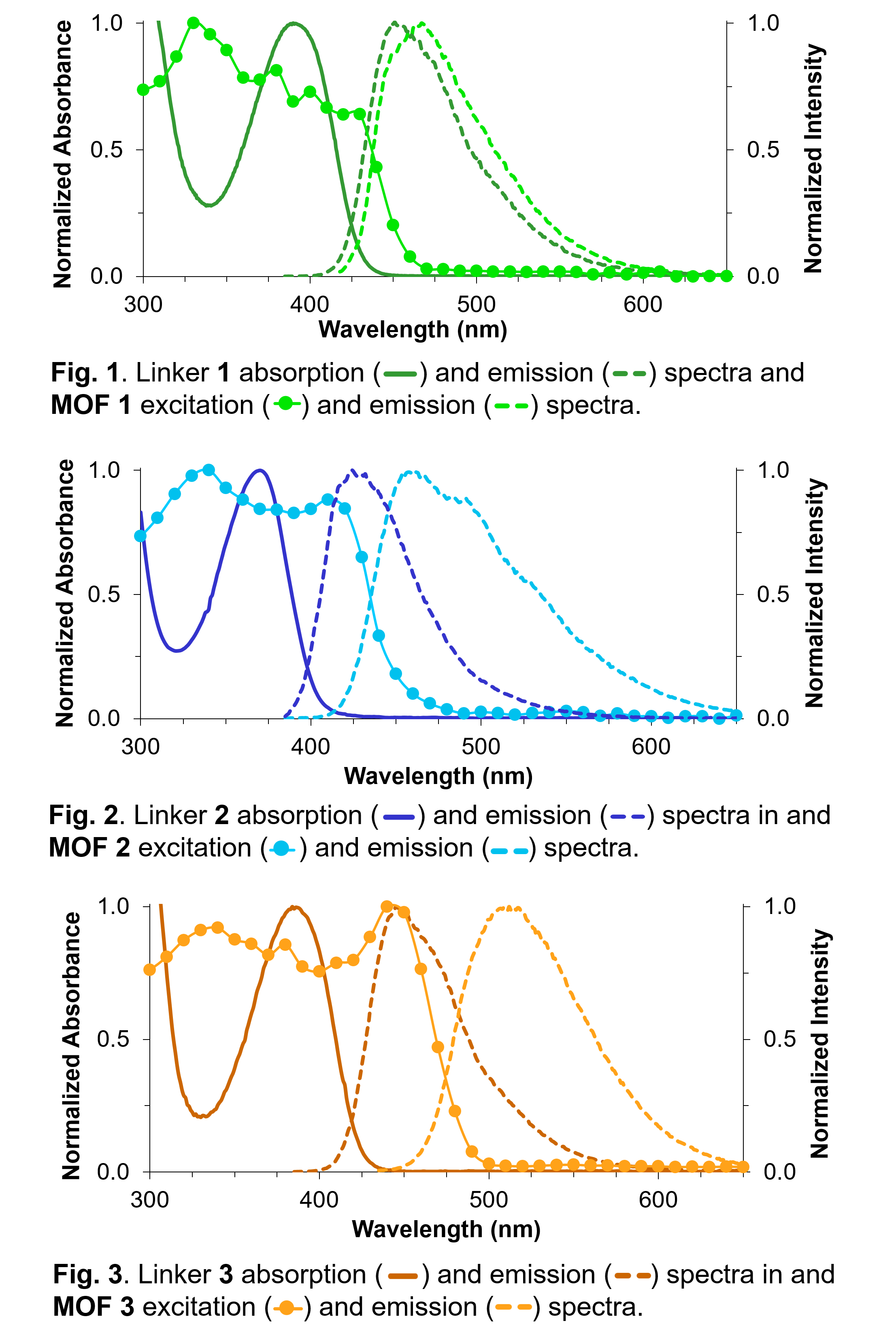
polymer. Structural modification of the linkers changes the photophysical properties of the MOFs. Using a variety of spectroscopies, we can characterize the fate of the electronically excited states within the MOFs.
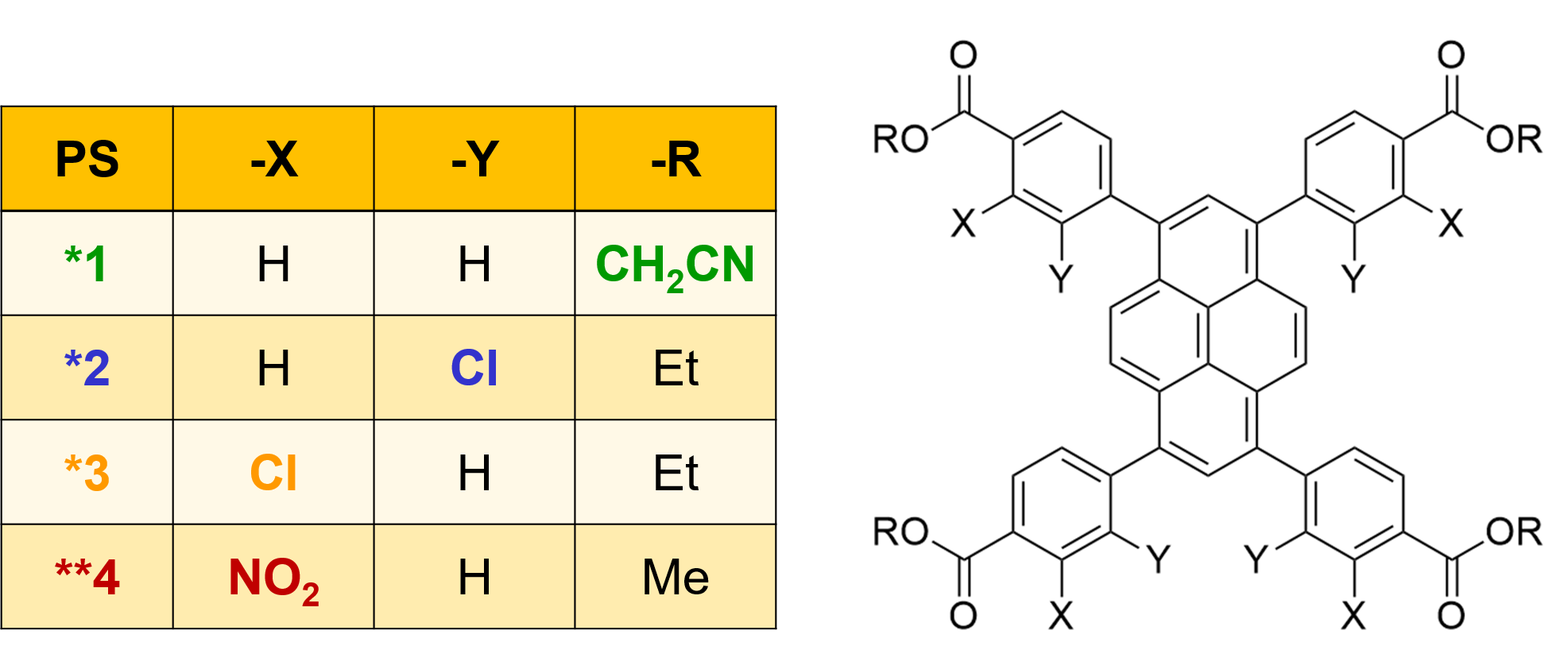
The photophysical deactivation processes are very much modified depending on the substituent pattern on the linker, as well as the MOF environment.
Finally, singlet oxygen can be quantified using the NIR phosphorescence of the oxidant.