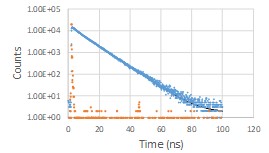
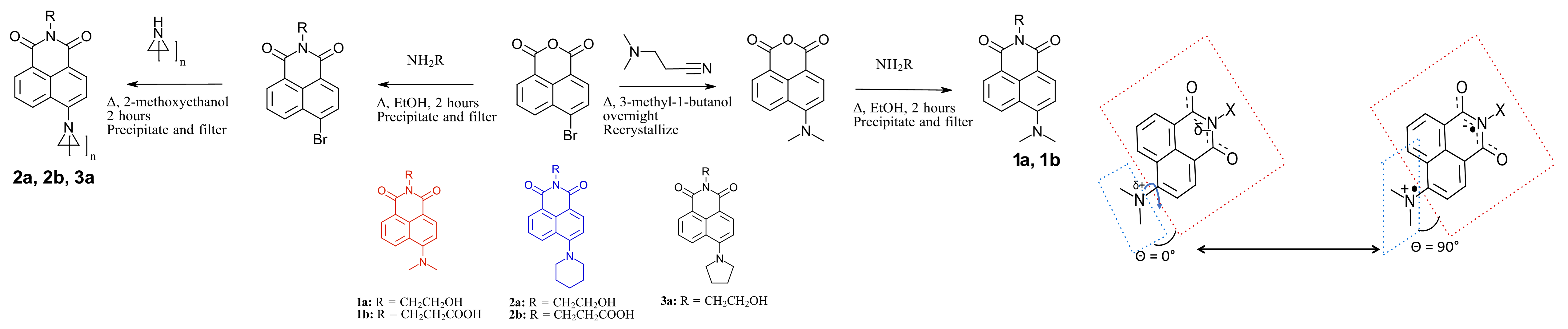
Substitution at the 4-position of 1,8-naphthalimides is well-known to create a solvent-dependent intramolecular charge transfer state (ICT). While this solvatochromism has been used in fluorescence imaging, the ability to tune the photochemistry (bond making/breaking) with solvent is largely unexplored.
Both the nature of the amine substituent at the 4-position, as well as the solvent (or polymer) environment the naphthalimides are in, impact the photochemistry. Both the inductive and steric factors are being studied.
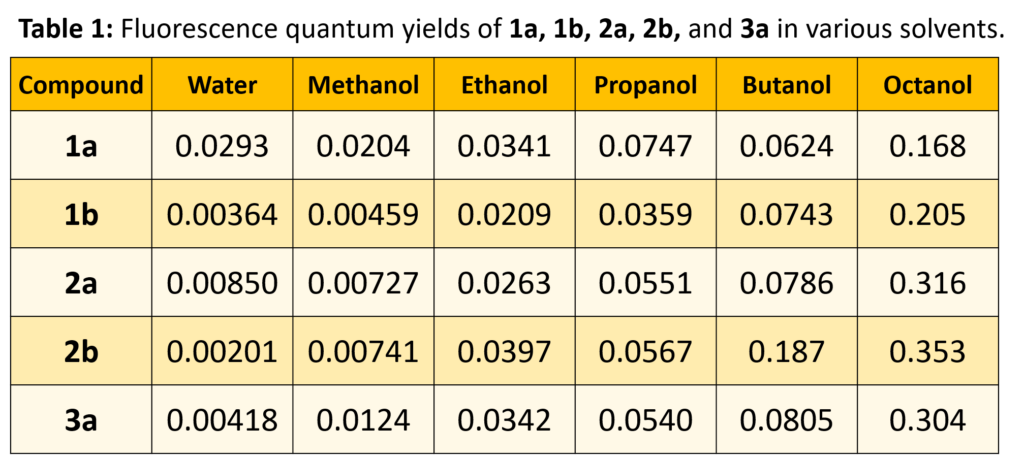
The fluorescence quantum yields, as well as excited-state dipole moments, are highly dependent on both solvent and substituent
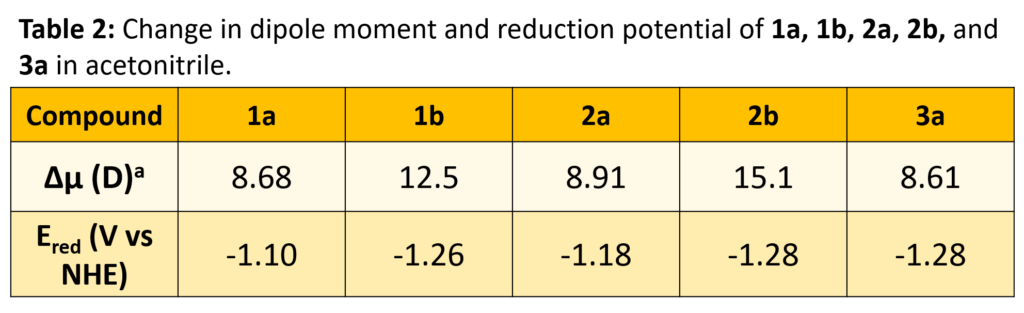
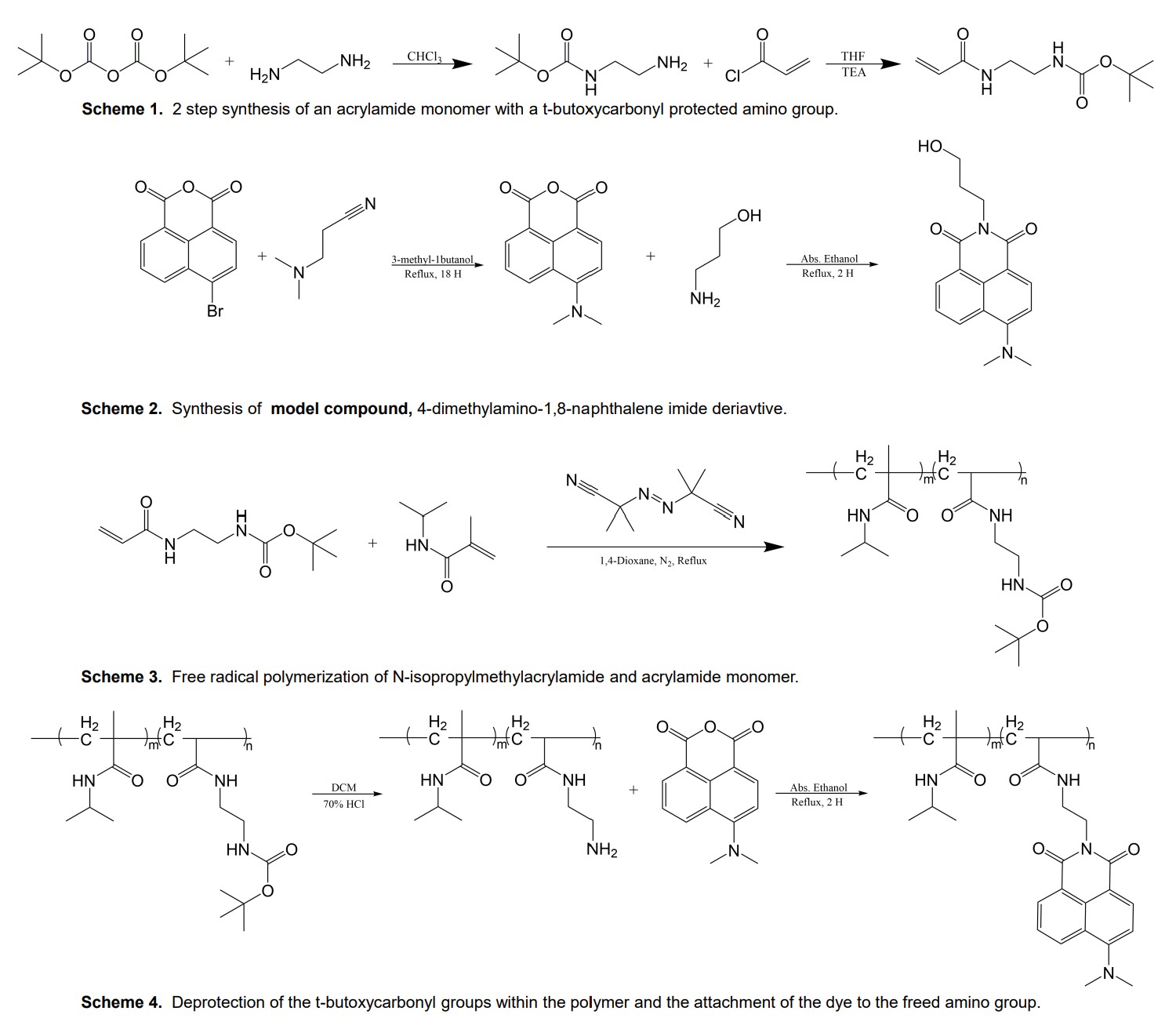
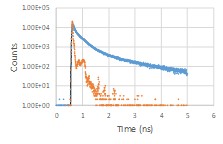
The nature of the excited state may be switched by solvent (aqueous vs. n-octanol):
Finally, stimuli-responsive polymers are well-known to change their hydrophobicity in response to an environmental stimulus. We are exploring how these polymers can be used to modify the photochemistry of these compounds.